Abstract
Background: Pts with LBCL that is refractory to or has relapsed after 1L therapy and who are not intended for HSCT have poor outcomes and limited treatment options. Liso-cel is an autologous CD19-directed CAR T cell product administered at equal target doses of CD8+ and CD4+ CAR+ T cells. In PILOT (NCT03483103), an open-label, single-arm, multicenter, phase 2 study in TNI pts with 2L R/R LBCL, liso-cel demonstrated high response rates and durable responses, with no new safety signals identified in 2L (Sehgal et al. Lancet Oncol 2022). In the current study, we assessed the comparative effectiveness of liso-cel in PILOT versus an external control cohort of pts with R/R LBCL who received conventional 2L chemotherapy regimens in the real-world clinical setting.
Methods: PILOT enrolled pts with R/R LBCL at any time after only 1L of chemoimmunotherapy containing an anthracycline and a CD20-targeted agent and with confirmed PET-positive disease, who were considered TNI by their physician, and who met ≥ 1 prespecified TNI criterion. The primary endpoint for PILOT was ORR. Secondary endpoints included CR rate, duration of response (DOR), PFS, event-free survival (EFS), and OS.
The real-world external control cohort was derived from a global, noninterventional, retrospective, observational study using multiple sources (COTA, Guardian Network, and clinical sites via electronic case report forms) representing pts with R/R LBCL across academic and community clinical settings in the US, Europe, and Japan. In the real world, intention to receive HSCT or not was not documented and was substituted with receipt of HSCT in this analysis. The real-world qualifying comparator cohort (QCC) included pts with R/R LBCL who received 1L treatment containing an anthracycline and CD20-targeted agent and any 2L treatment except HSCT and met PILOT eligibility criteria.
The primary endpoint for this study was ORR in the PILOT cohort versus the QCC. EFS was defined in this study as time from index date to first documentation of disease progression/relapse, start of new anticancer therapy, or any-cause death, whichever occurred first. The index and data cut-off dates, respectively, were the day of liso-cel infusion and 09/24/2021 for the PILOT cohort and the start of 2L therapy and 12/31/2020 for the QCC. The study period was Jul 2018 to Sept 2021. Endpoint analyses were performed with adjustment using the propensity score methodology of trimmed stabilized inverse probability of treatment weighting to balance pts in the QCC to the PILOT cohort according to baseline characteristics. Highly prognostic baseline characteristics (based on literature and medical review) with ≤ 30% missing values in both PILOT and the real-world cohort were included in balancing: age, sex, years from initial diagnosis to index date, ECOG performance status, Ann Arbor disease stage, refractory versus relapsed, duration of CR after 1L therapy, and bulky disease. All tests were conducted assuming a 2-tailed test of significance and alpha level set a priori at 0.05, with no adjustment for multiplicity.
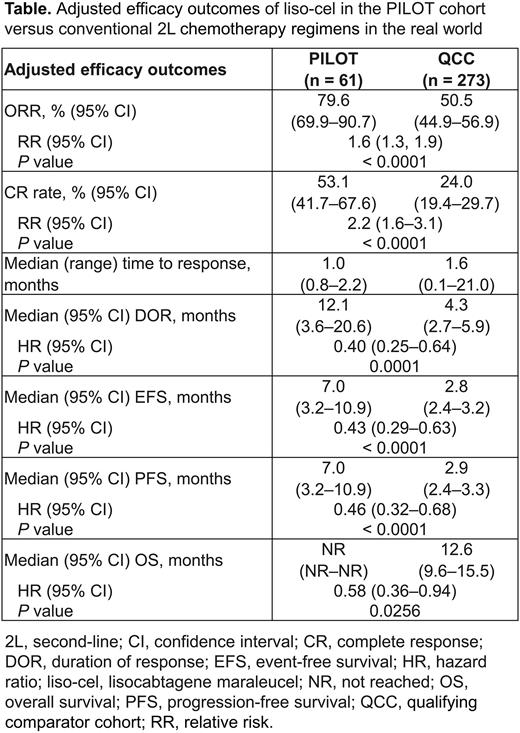
Results: In the PILOT cohort (n = 61) and QCC (n = 273), respectively, median (range) age was 74 (53‒84)/74 (21‒93) years, 79%/77% of pts were aged ≥ 70 years, 26%/24% had ECOG performance status of 2, 23%/8% had CrCl (Cockcroft and Gault) < 60 mL/min, 3%/2% had left ventricular ejection fraction < 50%, 2%/5% had aspartate aminotransferase > 2 × upper limit of normal (ULN), 0%/3% had alanine aminotransferase > 2 × ULN, and 66%/53% had Stage III or IV disease. In the QCC, the most common conventional 2L chemotherapy regimens were rituximab plus ifosfamide, carboplatin, and etoposide (R-ICE; 15%), bendamustine plus rituximab (12%), and rituximab plus gemcitabine and oxaliplatin (11%). Pts who received R-ICE might have been intended to proceed to HSCT but did not because of lack of response to R-ICE; however, intention to receive HSCT cannot be verified based on retrospective real-world data. There was a statistically significant difference in adjusted ORR, CR rate, median DOR, EFS, PFS, and OS in favor of the PILOT cohort versus the QCC (Table).
Conclusions: In pts with R/R LBCL who met prespecified TNI criteria, the difference in efficacy outcomes with liso-cel in PILOT versus conventional 2L chemotherapy regimens in the real world was statistically significant in favor of liso-cel, further supporting liso-cel as a new 2L therapy for pts with R/R LBCL who are not intended for HSCT.
Disclosures
Ghosh:Gilead, AstraZeneca, Bristol Myers Squibb, Phamacyclics, Janssen, Epizyme: Speakers Bureau; TG Therapeutics, Genentech/Roche, Bristol Myers Squibb, Gilead, Morphosys, AbbVie: Research Funding; Seagen, TG Therapeutics, AstraZeneca, Phamacyclics, Janssen, Bristol Myers Squibb, Gilead Sciences, Beigene, Incyte, Karyopharm, Roche/Genentech, Novartis, Loxo Oncology, Genmab, Adaptive Biotech, ADC Therapeutics: Consultancy. Sehgal:Kite, a Gilead Compnay: Other: Grants, Research Funding; Juno: Other: Grants, Research Funding; Milltenyiz: Research Funding; PeerView: Honoraria; OncLive: Honoraria. Liu:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Kostic:BMS: Current Employment, Current equity holder in publicly-traded company. Crotta:BMS: Current Employment, Current equity holder in publicly-traded company. Peng:BMS: Current Employment, Current equity holder in publicly-traded company. De Benedetti:BMS: Current Employment, Current equity holder in publicly-traded company. Gordon:Janssen: Other: DSMB; Ono Pharmaceuticals: Consultancy; Zylem: Current equity holder in private company, Current equity holder in publicly-traded company, Patents & Royalties: Patent on nanoparticles for lymphoma therapy; BMS: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal